How Do You Know if It Is Partially Compensated Uncompensated or Compensated
Interpretation of arterial blood gases (ABGs) is a crucial skill that a lot of student nurses and medical practitioners need to learn. In this guide, we'll help you understand the concepts backside arterial blood gas and teach yous the easiest and almost fun mode to interpret ABGs using the tic-tac-toe method.
What is arterial blood gas?

An arterial blood gas is a laboratory examination to monitor the patient's acid-base balance. It is used to determine the extent of the bounty by the buffer organisation and includes the measurements of the acidity (pH), levels of oxygen, and carbon dioxide in arterial blood. Unlike other blood samples obtained through a vein, a blood sample from an arterial blood gas (ABG) is taken from an artery (commonly on radial or brachial artery).
What are the components of arterial claret gas?
There are six components of arterial blood gas (ABGs):
pH
The pH is the concentration of hydrogen ions and determines the acerbity or alkalinity of body fluids. A pH of 7.35 indicates acidosis and a pH greater than seven.45 indicates alkalosis.The normal ABG level for pH is 7.35 to vii.45.
PaCO2 (Partial Pressure level of Carbon Dioxide)
PaCOtwo or partial pressure level of carbon dioxide shows the adequacy of the gas commutation betwixt the alveoli and the external environment (alveolar ventilation). Carbon dioxide (CO2) cannot escape when there is damage in the alveoli, excess CO2 combines with water to grade carbonic acrid (H2CO3) causing an acidotic state. When there is hypoventilation in the alveolar level (for case, in COPD), the PaCO2 is elevated, and respiratory acidosis results. On the other hand, when in that location is alveolar hyperventilation (e.k., hyperventilation), the PaCO2 is decreased causing respiratory alkalosis. For PaCO2, the normal range is 35 to 45 mmHg (respiratory determinant).
PaO2 (Partial Pressure of Oxygen)
PaO2 or partial force per unit area of oxygen or PAO2 indicates the amount of oxygen available to bind with hemoglobin. The pH plays a function in the combining power of oxygen with hemoglobin: a low pH means there is less oxygen in the hemoglobin. For PaO2, the normal range is 75 to 100 mmHg
SO2 (Oxygen Saturation)
SO2 or oxygen saturation, measured in percentage, is the corporeality of oxygen in the blood that combines with hemoglobin. Information technology can be measured indirectly by calculating the PAO2 and pH Or measured directly past co-oximetry. Oxygen saturation, the normal range is 94–100%
HCO3 (Bicarbonate)
HCOthree or bicarbonate ion is an alkaline substance that comprises over half of the total buffer base in the blood. A deficit of bicarbonate and other bases indicates metabolic acidosis. Alternatively, when there is an increment in bicarbonates present, and so metabolic alkalosis results.
BE (Base Backlog)
Exist. Base excess or BE value is routinely checked with HCO3 value. A base of operations excess of less than –2 is acidosis and greater than +2 is alkalosis. Base excess, the normal range is –2 to +2 mmol/50
Normal Values in Arterial Blood Gas
To determine acid-base imbalance, you need to know and memorize these values to recognize what deviates from normal. The normal range for ABGs is used as a guide, and the conclusion of disorders is often based on blood pH. If the blood is basic, the HCOthree level is considered considering the kidneys regulate bicarbonate ion levels. If the blood is acidic, the PaCOii or partial pressure of carbon dioxide in arterial blood is assessed because the lungs regulate the majority of acid. The normal ABG values are the post-obit:
- For pH, the normal range is seven.35 to 7.45
- For PaCOii, the normal range is 35 to 45 mmHg (respiratory determinant)
- For PaO2, the normal range is 75 to 100 mmHg
- For HCO3, the normal range is 22 to 26 mEq/L (metabolic determinant)
- Oxygen saturation, the normal range is 94–100%
- Base excess, the normal range is –2 to +2 mmol/L
Interpreting Arterial Blood Gas Imbalances
Interpreting arterial blood gases is used to detect respiratory acidosis or alkalosis, or metabolic acidosis or alkalosis during an acute disease. To determine the blazon of arterial claret gas the central components are checked. The all-time (and fun) manner of interpreting arterial blood gas is by using the tic-tac-toe method below:
Goals of Arterial Blood Gas assay

For the purpose of this guide, we have set three (3) goals that we need to achieve when interpreting arterial blood gases. The goals are as follows:
- Based on the given ABG values, determine if values translate ACIDOSIS or ALKALOSIS.
- 2d, we need to determine if values define METABOLIC or RESPIRATORY.
- Lastly, we need to determine the bounty if information technology is: FULLY COMPENSATED, PARTIALLY COMPENSATED, or UNCOMPENSATED.
We demand to go along these goals in mind as they'll come up later in the steps for the ABG interpretation technique.
Steps in ABG analysis using the tic-tac-toe method
In that location are eight (8) steps unproblematic steps you lot need to know if you want to translate arterial claret gases (ABGs) results using the tic-tac-toe technique.
one. Memorize the normal values.
The first step is you demand to familiarize yourself with the normal and abnormal ABG values when yous review the lab results. They are like shooting fish in a barrel to call up:
- For pH, the normal range is 7.35 to seven.45
- For PaCOii, the normal range is 35 to 45
- For HCO3, the normal range is 22 to 26
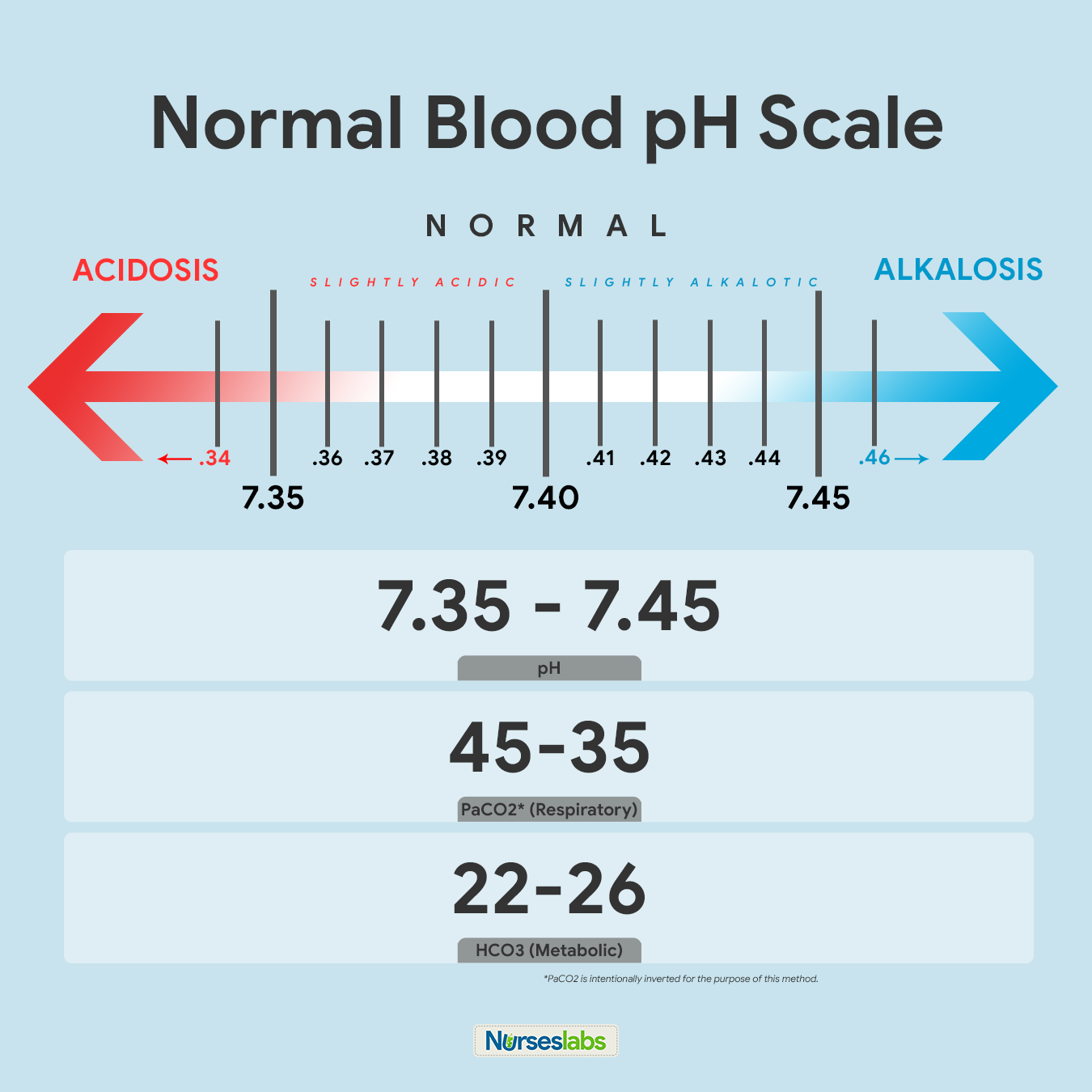
The recommended way of memorizing it is past drawing the diagram of normal values above. Write it downwards together with the arrows indicating ACIDOSIS or ALKALOSIS. Note that PaCO2 is intentionally inverted for the purpose of the Tic-Tac-Toe method.
two. Create your tic-tac-toe grid.
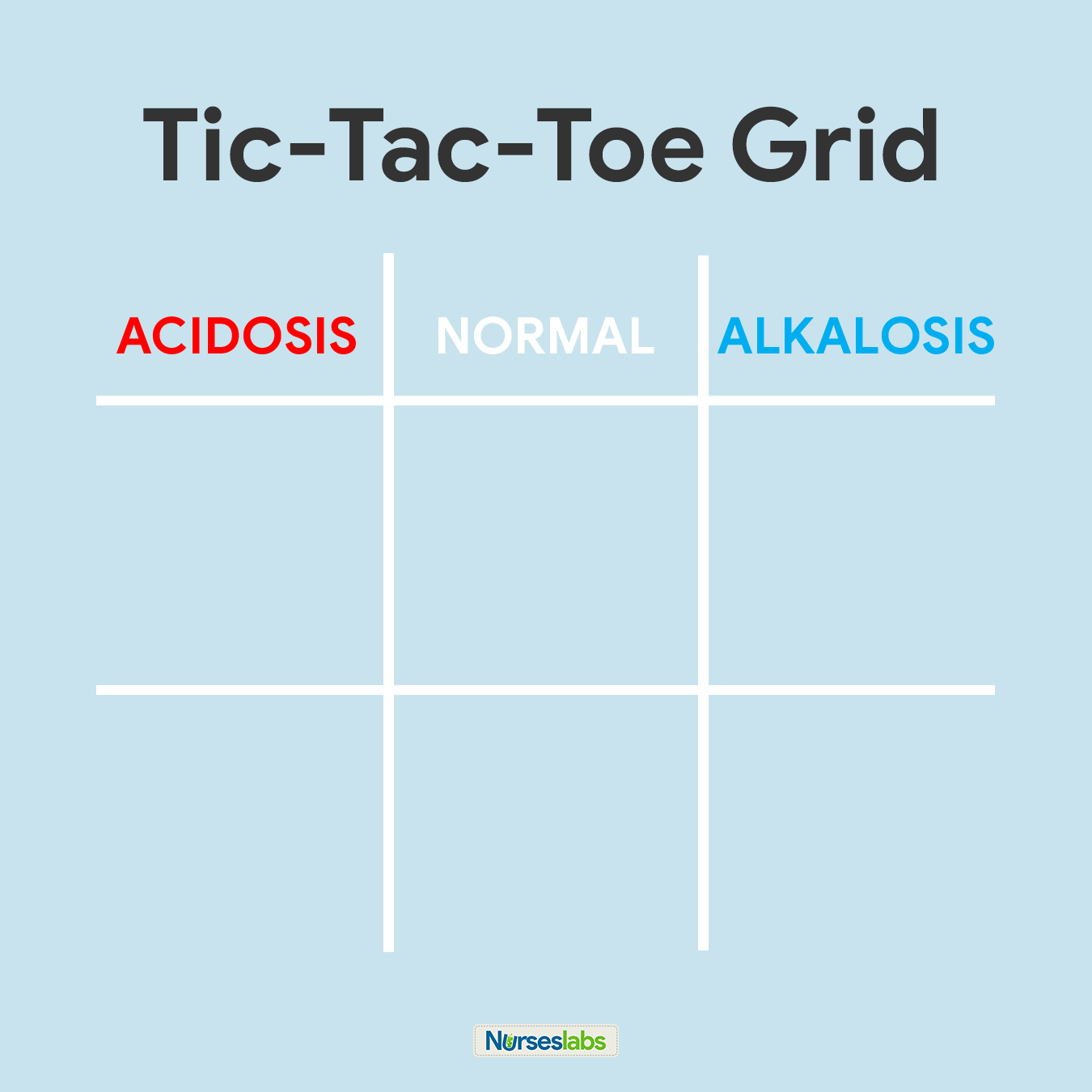
In one case you've memorized the normal values and the diagram, create a blank your tic-tac-toe grid and label the peak row as ACIDOSIS, NORMAL, and ALKALOSIS. Based on their values, nosotros need to determine in which column we'll place pH, PaCO2, and HCO3 in the grid.
3. Determine if pH is nether NORMAL, ACIDOSIS, or ALKALOSIS.
The third step of this technique is to determine the acidity or alkalinity of the blood with the given value of the pH as our determining factor. Call back in step #1 that the normal pH range is from 7.35 to 7.45.
- If the blood pH is betwixt seven.35 to 7.39, the interpretation is NORMAL but SLIGHTLY ACIDOSIS, place information technology under the NORMAL column.
- If the claret pH is between 7.41 to 7.45, interpretation is NORMAL but SLIGHTLY ALKALOSIS, place it nether the NORMAL column.
- Whatsoever blood pH below 7.35 (vii.34, 7.33, 7.32, and so on…) is ACIDOSIS, place it under the ACIDOSIS column.
- Whatsoever claret pH above 7.45 (vii.46, 7.47, 7.48, and then on…) is ALKALOSIS, place information technology under the ALKALOSIS column.
Please use the diagram below to help yous visualize whether the normal value is ACIDOSIS or ALKALOSIS.
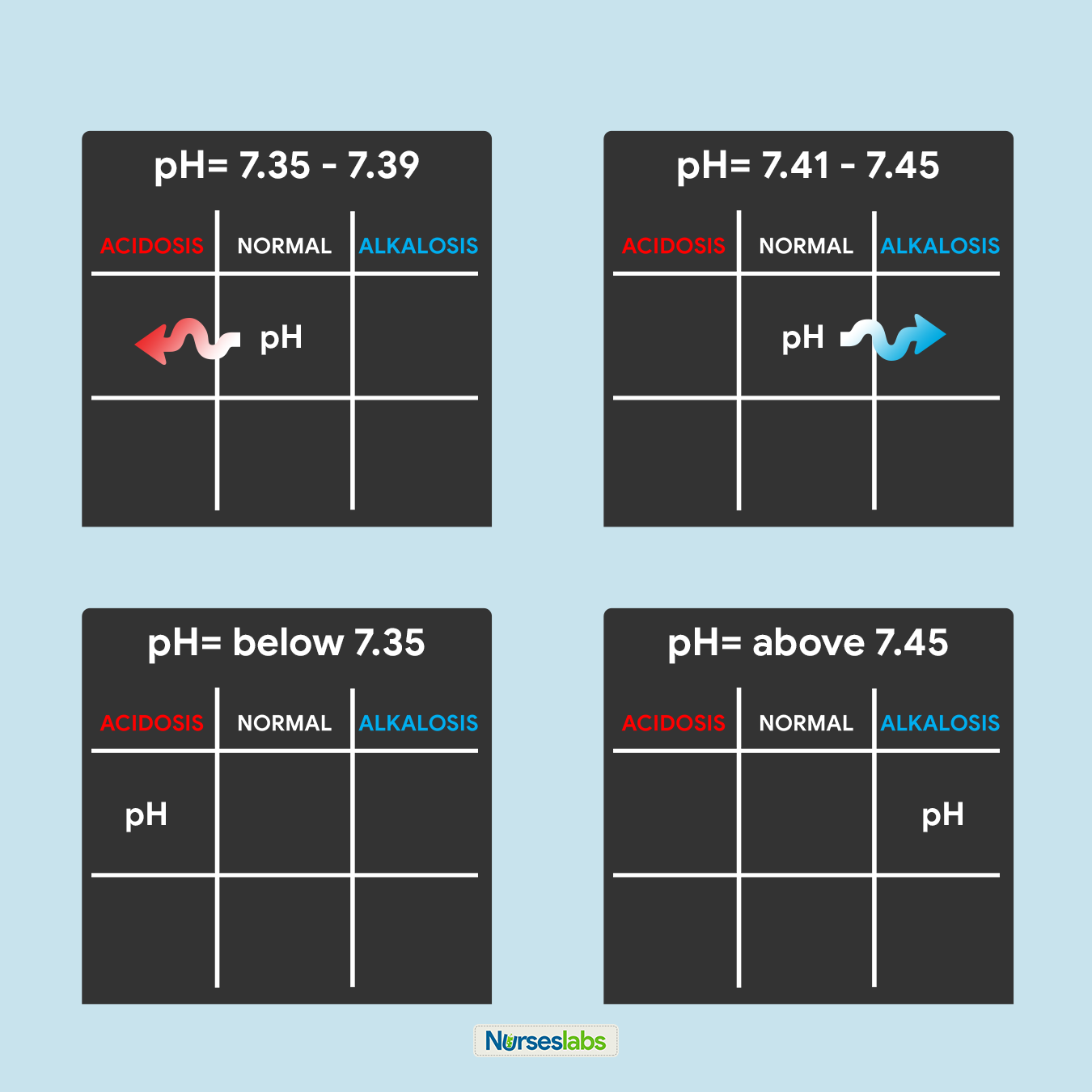
Once you've determined whether the pH is nether the ACIDOSIS or ALKALOSIS, plot information technology on your tic-tac-toe filigree under the advisable cavalcade.
4. Determine if PaCO2 is under NORMAL, ACIDOSIS, or ALKALOSIS.
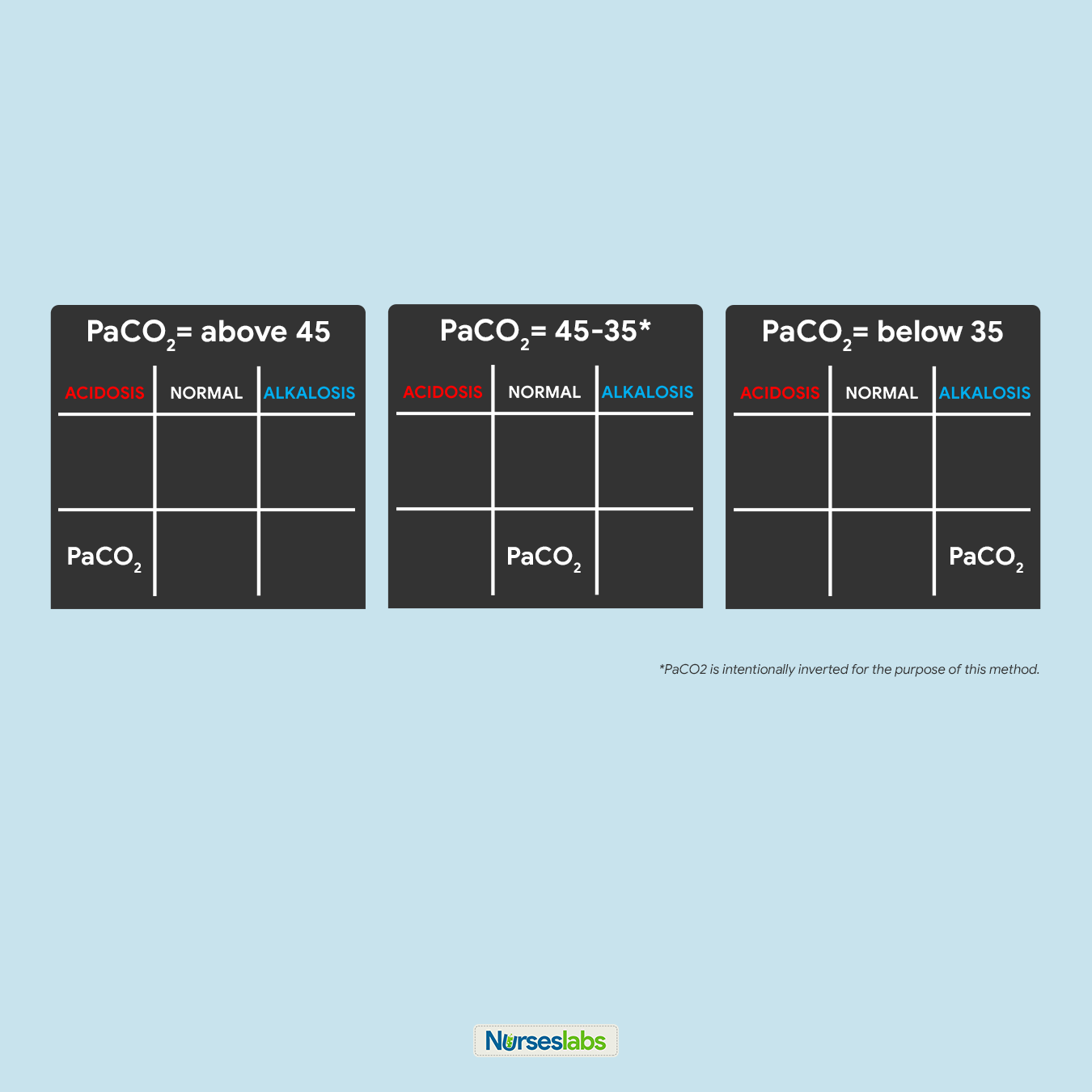
For this step, we need to interpret if the value of PaCO2 is within the NORMAL range, ACIDIC, or BASIC and plot it on the grid nether the advisable column. Recall that the normal range for PaCO2 is from 35 to 45:
- If PaCOtwo is below 35, place it under the ALKALOSIS cavalcade.
- If PaCO2 is in a higher place 45, identify it nether the ACIDOSIS column.
- If PaCO2 is within its normal range, place it under the NORMAL cavalcade.
five. Determine if HCO3 is under NORMAL, ACIDOSIS, or ALKALOSIS.

Next, nosotros need to interpret if the value of HCOthree is within the NORMAL range, ACIDIC, or Basic and plot it nether the appropriate column in the tic-tac-toe grid. Remember that the normal range for HCO3 is from 22 to 26:
- If HCOiii is below 22, place it under the ACIDOSIS column.
- If HCOthree is in a higher place 26, identify it under the ALKALOSIS cavalcade.
- If HCO3 is within its normal range, place it nether the NORMAL column.
6. Solve for goal #1: ACIDOSIS or ALKALOSIS.
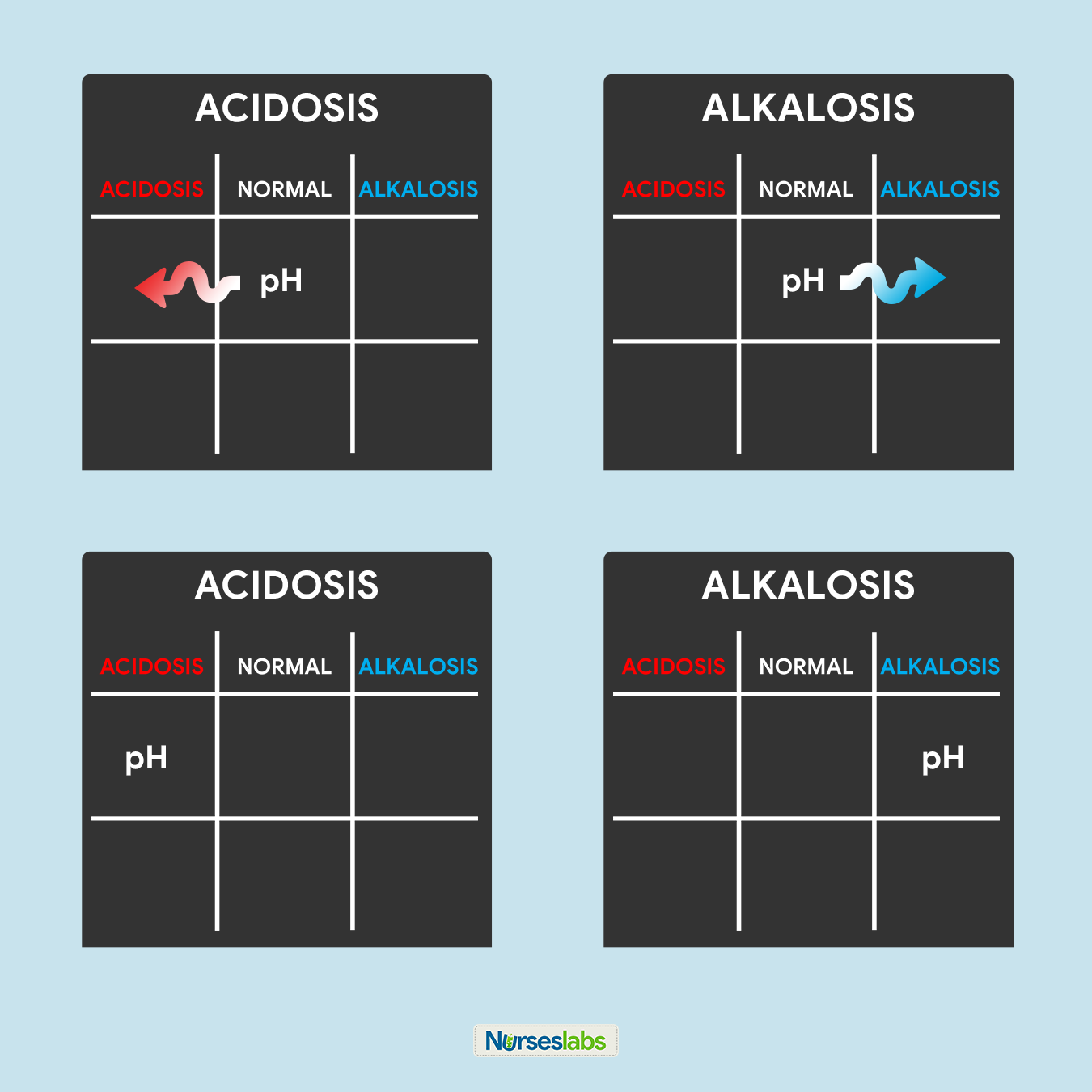
Now, we will start solving for our goals. Looking at the tic-tac-toe filigree, determine whether in what cavalcade the pH is placed and translate the results:
- If pH is under the ACIDOSIS column, it is ACIDOSIS.
- If pH is under the ALKALOSIS column, it is ALKALOSIS.
- If pH is under the NORMAL column, determine whether the value is leaning towards ACIDOSIS or ALKALOSIS and interpret appropriately.
In this pace, we tin can accomplish goal #ane of determining ACIDOSIS or ALKALOSIS.
7. Solve for goal #2: METABOLIC or RESPIRATORY.

Looking back over again on the tic-tac-toe grid, determine if pH is under the same column as PaCO2 or HCOthree and so we can achieve our goal #two of determining if the ABG is RESPIRATORY or METABOLIC. Interpret the results as follows:
- If pH is under the aforementioned column as PaCO2, it is RESPIRATORY.
- If pH is under the aforementioned column as HCOiii, it is METABOLIC.
- If pH is under the NORMAL column, determine whether the value is leaning towards ACIDOSIS or ALKALOSIS and translate accordingly.
8. Solve for goal #three: Compensation.
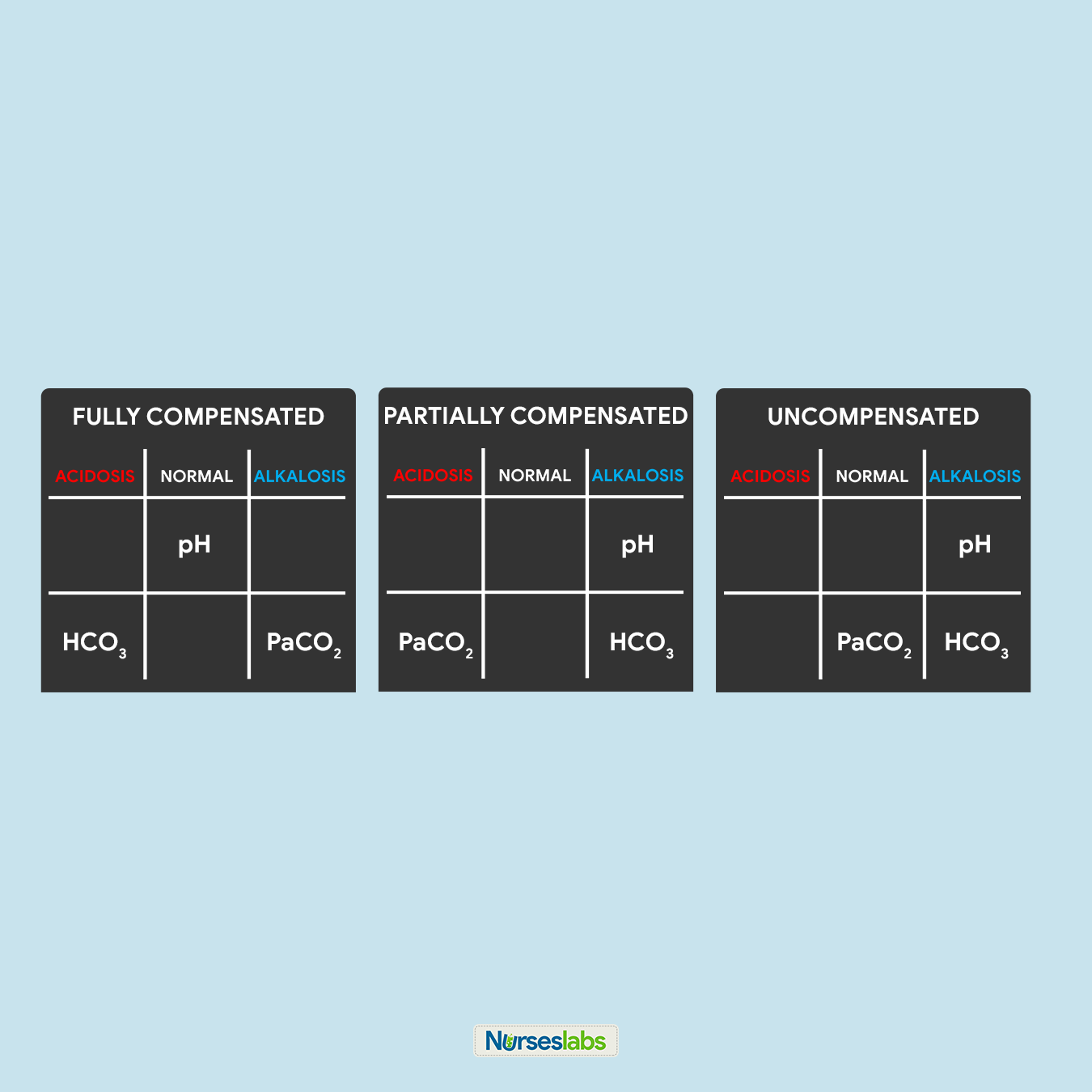
Lastly, we need to determine the compensation to accomplish our goal #iii. Interpret the results as follows:
- Information technology is FULLY COMPENSATED if pH is normal.
- It is PARTIALLY COMPENSATED if all three (3) values are aberrant.
- Information technology is UNCOMPENSATED if PaCOtwo or HCO3 is normal and the other is aberrant.
Awarding and Examples
Allow's solve for the ABG interpretation with the examples beneath:
Exercise Trouble #i:
pH=vii.26 | PaCOtwo=32 | HCO3=xviii
- Remember the normal values.
- Make your tic-tac-toe grid.
- pH of vii.26 Aberrant and under ACIDOSIS, so we place pH under ACIDOSIS.
- PaCOii of 32 is Abnormal and under ALKALOSIS, so we place PaCO2 under ALKALOSIS.
- HCO3 of 18 is ABNORMAL and under ACIDOSIS, then we place HCO3 under ACIDOSIS.
- pH is under ACIDOSIS, therefore solving for goal #1, we have ACIDOSIS.
- pH is on the same column equally HCOthree, therefore solving for goal #two, nosotros accept METABOLIC.
- All three values are ABNORMAL, therefore solving for goal #3, we accept a PARTIALLY COMPENSATED ABG.
The answer to Practise Problem #1:
Metabolic Acidosis, Partially Compensated
Practice Problem #ii:
pH=vii.44 | PaCO2=xxx | HCOthree=21
- Remember the normal values.
- Make your tic-tac-toe grid.
- pH of 7.44 is NORMAL simply slightly leaning towards ALKALOSIS, so nosotros place pH nether the NORMAL cavalcade with an arrow pointing towards the ALKALOSIS cavalcade.
- PaCOii of xxx is Abnormal and ALKALOSIS, then we place PaCO2 under the ALKALOSIS column.
- HCOiii of 21 is Abnormal and ACIDOSIS, so we place HCOiii under the ACIDOSIS column.
- pH of 7.44 is NORMAL but leaning towards ALKALOSIS, therefore solving for goal #1, nosotros have ALKALOSIS.
- pH is NORMAL but is leaning towards ALKALOSIS, therefore under the same column as PaCO2. Solving for goal #2, we accept RESPIRATORY.
- pH is NORMAL, therefore solving for goal #iii, we have a FULLY COMPENSATED ABG.
The answer to Practise Trouble #ii:
Respiratory Alkalosis, Fully Compensated
Do Problem #3:
pH=7.1 | PaCO2=xl | HCO3=18
- Remember the normal values.
- Make your tic-tac-toe filigree.
- pH of 7.one is Abnormal and ACIDOSIS, therefore, we place pH under the ACIDOSIS column in the tic-tac-toe grid.
- PaCOtwo of 40 is NORMAL, therefore, place it under the NORMAL column.
- HCO3 of 18 is ABNORMAL and ACIDOSIS, so nosotros place HCO3 nether the ACIDOSIS column.
- pH of seven.i is ACIDOSIS, therefore, solving for goal #1, we accept ACIDOSIS.
- pH is under the aforementioned column as HCO3, therefore, solving for goal #2, we have adamant that it is METABOLIC.
- pH is ABNORMAL and then as HCO3, but PaCO3 is under the NORMAL column. Solving for goal #3, we can translate information technology as UNCOMPENSATED.
The answer to Exercise Trouble #3:
Metabolic Acidosis, Uncompensated
How to depict Arterial Claret Gas?
Arterial claret is ordinarily drawn via the brachial or radial avenue.
- Inform that client about the procedure and that there is no food or fluid restriction imposed.
- Note if the client is taking anticoagulant therapy or aspirin as this may affect results.
- Note if the client is receiving oxygen therapy (flow rate, type of assistants device), and the client'due south electric current temperature.
- Using a heparinized needle and syringe, collect i to 5 mL of arterial blood. Mutual sites for drawing arterial blood are the radial and brachial artery.
- Put the syringe with arterial blood in an ice-water handbag to minimize the metabolic activity of the sample.
- Deliver the blood sample immediately to the laboratory.
- Employ pressure level to the puncture site for five minutes or longer.
Acid-Base Balance and Imbalances
Acid-base of operations imbalances develop when a person'southward normal homeostatic mechanisms are dysfunctional or overwhelmed. One type of acid-base imbalance is acidosis wherein the blood is relatively too acidic (low pH). The body produces two types of acid, therefore, at that place are two types of acidosis: respiratory acidosis and metabolic acidosis. On the contrary, alkalosis is a status wherein the claret is relatively too bones (high pH), there are besides ii types of alkalosis: respiratory alkalosis and metabolic alkalosis.
When acrid-base imbalances occur, the torso activates its compensatory mechanisms (the lungs and kidneys) to help normalize the blood pH. The kidneys compensate for respiratory acid-base imbalances while the respiratory arrangement compensates for metabolic acrid-base of operations imbalances. This does not correct the root cause of the problem, if the underlying condition is non corrected, these systems will fail.
Respiratory Acidosis
Respiratory acidosis occurs when breathing is inadequate (alveolar hypoventilation) and the lungs are unable to excrete enough CO2 causing PaCO2 or respiratory acrid builds up. The extra CO2 combines with water to form carbonic acid, causing a land of acidosis — a common occurrence in emphysema. The kidneys activate its compensatory procedure (admitting slow, frequently 24 hours or more) by increasing the excretion of metabolic acids through urination, which increases blood bicarbonate.
Types of Respiratory Acidosis
In that location are two forms of respiratory acidosis: Acute and Chronic.
- Acute respiratory acidosis. This grade of respiratory acidosis occurs immediately. Left untreated, symptoms will go progressively worse. It's a medical emergency and can become life-threatening.
- Chronic respiratory acidosis. This grade of respiratory acidosis develops through time. Information technology doesn't cause symptoms. Instead, the body adapts to the increased acidity. For case, the kidneys produce more than bicarbonate to aid maintain balance. Chronic respiratory acidosis may not cause symptoms. Developing another affliction may cause chronic respiratory acidosis to worsen and get acute respiratory acidosis.
Hazard Factors
Respiratory acidosis is typically caused past an underlying illness or condition. This is also called respiratory failure or ventilatory failure.
- Hypoventilation. A subtract in ventilation increases the concentration of carbon dioxide in the blood and decreases the claret's pH (brain trauma, blackout, hypothyroidism: myxedema).
- Chronic Obstructive Pulmonary Disease (COPD). In chronic respiratory acidosis in COPD patients, the body tries to recoup past retaining more bicarbonate to overcome acidosis.
- Respiratory Conditions. The lungs are non able to eliminate enough of the carbon dioxide produced past the torso. Excess carbon dioxide causes the pH of the blood and other bodily fluids to subtract, making them too acidic. (pneumothorax, pneumonia, condition asthmaticus)
- Drug Intake. Overdose of an opiate or opioid, such as morphine, tramadol, heroin, fentanyl, or magnesium sulfate (MgSO4) tin can cause respiratory acidosis.
Signs and Symptoms
Signs and symptoms of respiratory acidosis are as follows:
- Altered level of consciousness. Respiratory acidosis may be the outcome of an altered level of consciousness acquired by encephalopathy or cerebral edema.
- Confusion. Astute respiratory acidosis may as well cause symptoms involving the brain, including confusion, shock, drowsiness, and muscle jerks.
- Disorientation. Respiratory acidosis may result in disorientation, headache, or even focal neurologic signs.
- Coma. When the lungs tin can't remove all of the carbon dioxide produced by the trunk through normal metabolism, the blood becomes acidified, leading to increasingly serious symptoms, from sleepiness to coma.
- Tremors. Manifest as shaking or jerking muscle movements.
- Asterixis. An disability to maintain the posture of part of the body.
Direction of Respiratory Acidosis
Medical and nursing management of an arterial blood gas of respiratory acidosis includes the post-obit:
- Treat underlying conditions.
- Medications. Bronchodilator medicines and corticosteroids may be used to reverse some types of airway obstacle, like those linked to asthma and COPD.
- Weight loss. In the case of obesity hypoventilation syndrome, pregnant weight loss may exist necessary to reduce aberrant compression of the lungs.
- Provide mechanical ventilation through oxygen supplementation. Additional oxygen may be provided to convalesce the low oxygen level in the claret.
- Manage hyperkalemia through the utilise of Kayexalate. Acidosis causes potassium to movement from cells to extracellular fluid (plasma) in exchange for hydrogen ions, and alkalosis causes the reverse movement of potassium and hydrogen ions. Kayexalate increases fecal potassium excretion through the binding of potassium in the lumen of the alimentary canal.
- Maintain adequate hydration. Provide intravenous fluids and electrolytes as ordered.
Respiratory Alkalosis
Respiratory alkalosis can effect from hyperventilation since the lungs excrete too much carbonic acid which increases pH. Since respiratory alkalosis occurs quickly, the kidneys exercise not take time to compensate. Neurological symptoms such as confusion, paresthesias, and jail cell membrane excitability occur when the blood pH, CSF, and ICF increases acutely.
Take chances Factors
Causes of hyperventilation include:
- Panic. Panic attacks and anxiety are the about common causes of hyperventilation.
- Hyperthermia. Fever may manifest as hyperventilation. The exact mechanism is not known but is thought to be due to carotid body or hypothalamic stimulation past the increased temperature.
- Brainstem damage. Key neurogenic hyperventilation (CNH) is the human body's response to reduced carbon dioxide levels in the claret. This reduction in carbon dioxide is acquired past the contraction of cranial arteries from harm caused by lesions in the brain stem.
- Metabolic acidosis. Hyperventilation occurs about often as a response to hypoxia, metabolic acidosis, increased metabolic demands, pain, or anxiety.
- Diabetic ketoacidosis (DKA). The only known compensatory response to metabolic acidosis in DKA is hyperventilation with consecutive respiratory alkalosis.
- Pregnancy. Progesterone levels are increased during pregnancy. Progesterone causes stimulation of the respiratory middle, which tin can atomic number 82 to respiratory alkalosis.
- Salicylate toxicity. Salicylate toxicity causes respiratory alkalosis and, by an independent mechanism, metabolic acidosis.
Signs and Symptoms
Hyperventilation is a sign that respiratory alkalosis is most probable to occur. Still, depression carbon dioxide levels in the blood too have a number of concrete effects, including:
- Numbness. Increased neuromuscular irritability in which a person loses feeling in a item role of their body.
- Tingling sensation. Prickling awareness that is ordinarily felt in the hands, arms, legs, or feet, merely can as well occur in other parts of the torso.
- Palpitations. Palpitations are the perceived aberration of the heartbeat characterized by sensation of cardiac muscle contractions in the chest.
- Tetany. Tetany or tetanic seizure is a medical sign consisting of the involuntary contraction of muscles.
- Convulsions. A medical condition where torso muscles contract and relax rapidly and repeatedly, resulting in uncontrolled actions of the body.
- Signs and symptoms of hypokalemia and hypocalcemia. Persistent respiratory alkalosis can induce secondary hypocalcemia and hypokalemia that may crusade cardiac arrhythmias, conduction abnormalities, and diverse somatic symptoms such as paresthesia, hyperreflexia, convulsive disorders, muscle spasm, muscle twitching, positive Chvostek'southward sign, and tetany.
Direction of Respiratory Alkalosis
The handling for respiratory alkalosis depends on the underlying crusade. Treating the status is a matter of rising carbon dioxide levels in the blood. The following strategies and tips are useful for respiratory alkalosis acquired by over-animate due to panic and anxiety.
- Breathe into a newspaper purse. Breathing through a paper bag fills it with carbon dioxide helping in inhaling exhaled air dorsum into the lungs.
- Care for underlying condition:
- Medications. Administering an opioid pain reliever or anti-anxiety medication to reduce hyperventilation.
- Relaxation techniques. Breathing exercises that aid relax and breathe from the diaphragm and abdomen, rather than chest wall.
- Prophylactic. Stay with the patient.
- Lavage. After massive aspirin ingestions, ambitious gut decontamination is advisable, including gastric lavage.
- Correction of hypokalemia and hypocalcemia.
- Oxygenation as indicated. Providing oxygen to help keep a person from hyperventilating.
Metabolic Acidosis
Metabolic acidosis is when there is a subtract in bicarbonates and a buildup of lactic acid occurs. This happens in diarrhea, ketosis, and kidney disorders. It has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids.
Take chances Factors
- Diabetic Ketoacidosis (DKA). DKA develops when substances chosen ketone bodies (which are acidic) build up during uncontrolled diabetes. DKA occurs mostly in Type 1 Diabetes Mellitus (DM).
- Chronic Renal Failure (CRF). This is due to reduced tubular bicarbonate reabsorption and bereft renal bicarbonate production in relation to the number of acids synthesized by the body and ingested with food.
- Chronic Hypoxia. With chronic hypoxia, metabolic and hypercapnic acidosis develop along with considerable lactate germination and pH falling to below vi.eight.
- Obesity. Obesity, especially in conjunction with insulin resistance, can increase metabolic acidosis and thus upshot in a reduction of urinary citrate excretion.
- Diarrhea. Loss of bicarbonate stores through diarrhea or renal tubular wasting leads to a metabolic acidosis state characterized past increased plasma chloride concentration and decreased plasma bicarbonate concentration.
- Dehydration. Electrolyte disturbances caused by prolonged vomiting or severe dehydration tin can cause metabolic acidosis.
- Aspirin Toxicity. Aspirin overdose causes the trunk to non produce ATP, leading to anaerobic metabolism with consequent raised lactate and ketone bodies. Astute aspirin or salicylates overdose or poisoning can crusade initial respiratory alkalosis through metabolic acidosis ensues thereafter.
- Methanol Poisoning. Significant methanol ingestion leads to metabolic acidosis, which is manifested by a low serum bicarbonate level. The anion gap is increased secondary to high lactate and ketone levels. This is probably due to formic acrid accumulation.
Signs and Symptoms
- Altered level of consciousness
- Defoliation
- Disorientation
- Lack of ambition
- Coma
- Jaundice
Management of Metabolic Acidosis
Patients with arterial blood gas indicating metabolic acidosis are managed and treated by:
- Sodium bicarbonate. Indicated in the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes, circulatory insufficiency due to shock or astringent dehydration, extracorporeal circulation of blood, cardiac abort, and severe master lactic acidosis.
- Treat the underlying condition.
- Hydration for diabetic ketoacidosis. The major treatment of this condition is the initial rehydration.
- Dialysis for chronic renal failure. The control of metabolic acidosis in hemodialysis is mainly focused on the supply of bicarbonate during the dialysis sessions.
- Use of diuretics.
- Initiate condom measures.
- Kayexalate. Acidosis causes potassium to motility from cells to extracellular fluid (plasma) in exchange for hydrogen ions, and alkalosis causes the reverse movement of potassium and hydrogen ions. Kayexalate increases fecal potassium excretion through the binding of potassium in the lumen of the gastrointestinal tract.
Metabolic Alkalosis
Metabolic alkalosis occurs when bicarbonate ion concentration increases, causing an peak in claret pH. This tin occur in excessive vomiting, dehydration, or endocrine disorders.
Risk Factors
- Vomiting. Vomiting causes metabolic alkalosis by the loss of gastric secretions, which are rich in muriatic acid (HCl). Whenever a hydrogen ion is excreted, a bicarbonate ion is gained in the extracellular space.
- Sodium bicarbonate overdose. Administration of sodium bicarbonate in amounts that exceed the capacity of the kidneys to excrete this excess bicarbonate may cause metabolic alkalosis.
- Hypokalemia. Due to a low extracellular potassium concentration, potassium shifts out of the cells. In order to maintain electrical neutrality, hydrogen shifts into the cells, raising blood pH.
- Nasogastric suction. Just like in airsickness, nasogastric (NG) suction also generates metabolic alkalosis by the loss of gastric secretions, which are rich in muriatic acid (HCl).
Signs and Symptoms
Metabolic alkalosis may not show whatsoever symptoms. People with this type of alkalosis more than often mutter of the underlying conditions that are causing information technology. These tin can include:
- Numbness
- Vomiting
- Diarrhea
- Swelling in the lower legs (peripheral edema)
- Fatigue
- Tingling awareness
- Agitation
- Disorientation
- Seizures
- Blackout
Management of Metabolic Alkalosis
- Antiemetic. In the case of vomiting, administrate antiemetics, if possible.
- Ammonium chloride. Ammonium chloride is a systemic and urinary acidifying agent that is converted to ammonia and muriatic acid through oxidation by the liver. Intravenous (IV) ammonium chloride is a treatment selection for severe cases of metabolic alkalosis.
- Acetazolamide (Diamox). Acetazolamide also appears to be safe and constructive in patients with metabolic alkalosis post-obit treatment of respiratory acidosis from exacerbations of chronic obstructive pulmonary illness (COPD).
Arterial Blood Gas Interpretation Quiz
If you need to practice your new skills acquired here, cheque out our Arterial Blood Gas Estimation for NCLEX (40 Questions)
References and Sources
The following sources are used every bit references for this guide. You may find them interesting for your additional reading:
- Barnette, Fifty., & Kautz, D. D. (2013). Artistic ways to teach arterial blood gas interpretation.Dimensions of Critical Care Nursing,32(2), 84-87.
- Samuel, R. (2018). A Graphical Tool for Arterial Claret Gas Estimation using Standard Bicarbonate and Base of operations Excess.Indian J Med Biochem,22(1), 85-89.
- Sood, P., Paul, G., & Puri, S. (2010). Interpretation of arterial blood gas.Indian journal of critical intendance medicine: peer-reviewed, official publication of Indian Gild of Disquisitional Care Medicine,14(2), 57.
- Williams, A. J. (1998). Assessing and interpreting arterial claret gases and acrid-base balance.Bmj,317(7167), 1213-1216.
- Verma, A. G., & Roach, P. (2010). The interpretation of arterial blood gases.Aust Prescr,33(4), 124-129.
christiansenhatabligh.blogspot.com
Source: https://nurseslabs.com/arterial-blood-gas-abgs-interpretation-guide/
0 Response to "How Do You Know if It Is Partially Compensated Uncompensated or Compensated"
Postar um comentário